Gallery
 PM Modi visit USA
PM Modi visit USA Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan
Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan Karnataka rain fury: Photos of flooded streets, uprooted trees
Karnataka rain fury: Photos of flooded streets, uprooted trees Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit
Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss
Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss Oscars 2022: Every Academy Award Winner
Oscars 2022: Every Academy Award Winner Shane Warne (1969-2022): Australian cricket legend's life in pictures
Shane Warne (1969-2022): Australian cricket legend's life in pictures Photos: What Russia's invasion of Ukraine looks like on the ground
Photos: What Russia's invasion of Ukraine looks like on the ground Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India'
Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India' PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)
PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)India Open Competition in Shotgun, organised by the National Rifle Association of India (N
- Hockey India names Amir Ali-led 20-man team for Junior Asia Cup
- Harmanpreet Singh named FIH Player of the Year, PR Sreejesh gets best goalkeeper award
- World Boxing medallist Gaurav Bidhuri to flag off 'Delhi Against Drugs' movement on Nov 17
- U23 World Wrestling Championship: Chirag Chikkara wins gold as India end campaign with nine medals
- FIFA president Infantino confirms at least 9 African teams for the 2026 World Cup
Coronavirus Vaccine: Baird analysts find gaps in positive results from remdesivir trial Last Updated : 18 Apr 2020 08:14:11 AM IST 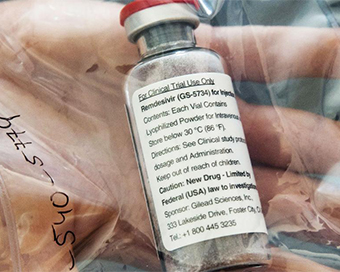
Remdesivir After shares of pharmaceutical major Gilead Sciences surged following a media report that the company's antiviral drug remdesivir showed promise in treating coronavirus patients in a "closely watched clinical trial", analysts at Robert W. Baird & Co. said that the results need to be critically looked into.
According to a report in the health-oriented news website STAT, most of the patients recruited for the studies reported fast recoveries in fever and respiratory symptoms.Following the treatment almost all of them were discharged in less than a week, while only two died, according to the report.But in an investment research report, the Baird analysts pointed out that the results reported by STAT were based on "uncontrolled, anecdotal data, which often winds up not being confirmed in controlled studies".STAT did say that all the patients in the trials were given remdesivir, suggesting that there were no control populations.Moreover, the results of the studies were leaked.An infectious disease specialist at University of Chicago discussed the trial results with other University of Chicago faculty members. STAT obtained a copy of the recorded conversation.The Baird analysts said that these results need to be cautiously interpreted and added that the more definitive answer will come from the double-blind placebo controlled study by National Institute of Allergy and Infectious Diseases (NIAID), part of the US National Institutes of Health, expected in late May.Remdesivir is an investigational broad-spectrum antiviral treatment. It was previously tested in humans with Ebola virus disease and has shown promise in animal models for treating Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), which are caused by other coronaviruses.The safety and efficacy of remdesivir to treat coronavirus are being evaluated in multiple ongoing Phase 2 and 3 clinical trials.Gilead earlier said that a study in China in patients with severe disease was terminated early due to low enrollment.But the study in China in patients with mild-to-moderate disease is ongoing, it added.Additional studies of remdesivir and other investigational treatments for Covid-19, based on a master protocol by the World Health Organization, have also begun to enroll patients in countries around the world.IANS San Francisco For Latest Updates Please-
Join us on
Follow us on








172.31.16.186