Gallery
 PM Modi visit USA
PM Modi visit USA Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan
Only the mirror in my washroom and phone gallery see the crazy me : Sara Khan Karnataka rain fury: Photos of flooded streets, uprooted trees
Karnataka rain fury: Photos of flooded streets, uprooted trees Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit
Cannes 2022: Deepika Padukone stuns at the French Riviera in Sabyasachi outfit Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss
Ranbir Kapoor And Alia Bhatt's Wedding Pics - Sealed With A Kiss Oscars 2022: Every Academy Award Winner
Oscars 2022: Every Academy Award Winner Shane Warne (1969-2022): Australian cricket legend's life in pictures
Shane Warne (1969-2022): Australian cricket legend's life in pictures Photos: What Russia's invasion of Ukraine looks like on the ground
Photos: What Russia's invasion of Ukraine looks like on the ground Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India'
Lata Mangeshkar (1929-2022): A pictorial tribute to the 'Nightingale of India' PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)
PM Modi unveils 216-feet tall Statue of Equality in Hyderabad (PHOTOS)India Open Competition in Shotgun, organised by the National Rifle Association of India (N
- Hockey India names Amir Ali-led 20-man team for Junior Asia Cup
- Harmanpreet Singh named FIH Player of the Year, PR Sreejesh gets best goalkeeper award
- World Boxing medallist Gaurav Bidhuri to flag off 'Delhi Against Drugs' movement on Nov 17
- U23 World Wrestling Championship: Chirag Chikkara wins gold as India end campaign with nine medals
- FIFA president Infantino confirms at least 9 African teams for the 2026 World Cup
First patients injected in UK coronavirus vaccine trial Last Updated : 24 Apr 2020 09:24:13 AM IST 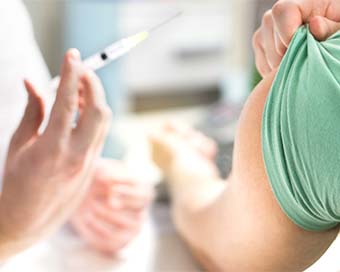
file photo The first human trial in Europe of a coronavirus vaccine has begun in Oxford.
Two volunteers were injected, the first of more than 800 people recruited for the study, the BBC reported.Half will receive the COVID-19 vaccine, and half a control vaccine which protects against meningitis but not coronavirus.The design of the trial means volunteers will not know which vaccine they are getting, though doctors will.Elisa Granato, one of the two who received the jab, told the BBC: "I'm a scientist, so I wanted to try to support the scientific process wherever I can."The vaccine was developed in under three months by a team at Oxford University. Sarah Gilbert, professor of vaccinology at the Jenner Institute, led the pre-clinical research."Personally I have a high degree of confidence in this vaccine," she said."Of course, we have to test it and get data from humans. We have to demonstrate it actually works and stops people getting infected with coronavirus before using the vaccine in the wider population."Prof Gilbert previously said she was "80% confident" the vaccine would work, but now prefers not to put a figure on it, saying simply she is "very optimistic" about its chances.The vaccine is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees that has been modified so it cannot grow in humans.The Oxford team has already developed a vaccine against Mers, another type of coronavirus, using the same approach - and that had promising results in clinical trials.The only way the team will know if the Covid-19 vaccine works is by comparing the number of people who get infected with coronavirus in the months ahead from the two arms of the trial.That could be a problem if cases fall rapidly in the UK, because there may not be enough data.Prof Andrew Pollard, director of the Oxford Vaccine Group, who is leading the trial, said: "We're chasing the end of this current epidemic wave. If we don't catch that, we won't be able to tell whether the vaccine works in the next few months. But we do expect that there will be more cases in the future because this virus hasn't gone away."The vaccine researchers are prioritising the recruitment of local healthcare workers into the trial as they are more likely than others to be exposed to the virus.A larger trial, of about 5,000 volunteers, will start in the coming months and will have no age limit.The first human trial in Europe of a coronavirus vaccine has begun in Oxford.Two volunteers were injected, the first of more than 800 people recruited for the study, the BBC reported.Half will receive the COVID-19 vaccine, and half a control vaccine which protects against meningitis but not coronavirus.The design of the trial means volunteers will not know which vaccine they are getting, though doctors will.Elisa Granato, one of the two who received the jab, told the BBC: "I'm a scientist, so I wanted to try to support the scientific process wherever I can."The vaccine was developed in under three months by a team at Oxford University. Sarah Gilbert, professor of vaccinology at the Jenner Institute, led the pre-clinical research."Personally I have a high degree of confidence in this vaccine," she said."Of course, we have to test it and get data from humans. We have to demonstrate it actually works and stops people getting infected with coronavirus before using the vaccine in the wider population."Prof Gilbert previously said she was "80% confident" the vaccine would work, but now prefers not to put a figure on it, saying simply she is "very optimistic" about its chances.The vaccine is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees that has been modified so it cannot grow in humans.The Oxford team has already developed a vaccine against Mers, another type of coronavirus, using the same approach - and that had promising results in clinical trials.The only way the team will know if the Covid-19 vaccine works is by comparing the number of people who get infected with coronavirus in the months ahead from the two arms of the trial.That could be a problem if cases fall rapidly in the UK, because there may not be enough data.Prof Andrew Pollard, director of the Oxford Vaccine Group, who is leading the trial, said: "We're chasing the end of this current epidemic wave. If we don't catch that, we won't be able to tell whether the vaccine works in the next few months. But we do expect that there will be more cases in the future because this virus hasn't gone away."The vaccine researchers are prioritising the recruitment of local healthcare workers into the trial as they are more likely than others to be exposed to the virus.A larger trial, of about 5,000 volunteers, will start in the coming months and will have no age limit.IANS London For Latest Updates Please-
Join us on
Follow us on








172.31.16.186